Food poisoning microorganisms
Here's some additional support sites for specific foodborne pathogens. The first section is a series of quick links.
- WHO fact sheet
- FDA Bad Bug book
- Genome sequence
- Guillain-Barre Syndrome (GBS)
- Biofilm production under oxygen stress conditions
Campylobacter jejuni sites
- FDA Bad Bug book
- Multiple antibiotic resistance, WHO factsheet
- Enter-Net surveillance, EU
- Salmonella (and other causes of gastroenteritis) HPA (England & Wales)
- Salmonella in poultry (UK)
- EU Scientific Committee opinion on salmonellae in foodstuffs
- Outbreak associated with eggs
- Coming to grips with outbreaks in US
- Multiple resistant strains from pet rodents
- Molecular subtyping
- Salmonella cost estimate in USA
- Household contamination
- Household contamination">To eat peanut butt, or not to eat ? That is the question
- PulseNet
- News on food poisoning
- Salmonella genome sequence, Nature paper
Salmonella serovars
- E. coli O104:H4 outbreak May-June 2011 Germany
- CDC - E. coli O157
- CDC - EHEC
- CDC - ETEC
- Toxins
- STEC outbreak in Wales; Secondary transmission case study
- Outbreak investigation in Japan
- E. coli O111 outbreak case study
- E. coli various serotypes antibiotic resistance study
- E. coli 026 case in Korea
- Genome sequence E. coli O157
- HUS and HC surveillance
- International outbreak due to lettuce, 2007
- Pennington Group report in the the Lanarkshire outbreak
- FoodNet (USA) surveillance
- PulseNet
- Non-O157 STEC survey, Nebraska (CDC)
Escherichia coli sites
- General background, FDA Bad Bug book
- Outbreak
- Lecture
- Genomic sequence
Clostridium perfringens sites
- FDA Bad Bug book
- Detection in RTE food processing environment
- Multistate outbreak 2000
- Listeriosis increase in Denmak, 2009
- FAO-WHO MRA for ready-to-eat foods
- Genome sequence
- L. monocytogenes seroptype 4b - evolutionary intermediate
- MLST L. innocua - 4 subgroups
- Genome comparison of two outbreak L. monocytogenes 1/2a outbreak strains
Listeria monocytogenes sites
- General background
- Cruise ship outbreaks
- Cruise ship outbreak investigation
- Foodborne viruses; emerging problem
- Foodborne viruses - review
- Detection method
- Monitoring
- Two Epidemiologic Patterns of Norovirus Outbreaks: Surveillance in England and Wales, 1992-2000
- Viral Gastroenteritis Outbreaks in Europe, 1995-2000
- Multi-country European outbreak in 2010
- Double outbreak
- Fat Duck payout
- UK surveillance study data
- EU Scientific Committee opinion (pdf file)
Noroviruses (formerly Norwalk-like viruses and small round structured viruses), and other enteric viruses
Cronobacter (Enterobacter sakazakii)
Although the primary concern with this bacterium is the association with neonatal infections, the majority of cases are in adults. The bacterium was previously referred at as a 'yellow-pigmented Enterobacter cloacae' This is unfortunate as more recent studies have shown that ~5-7% of strains are non-pigmented. Hence initial identification methods based on pigmentation (FDA and ISO) could fail to isolate the bacterium.
See www.foodmicrobe.com and click on 'Publications' for a full up to date list of papers from our research group. These publications include:
- Multilocus sequence typing revealing predominant sequence type associated with neonatal meningitis
- UK FSA report on bacteriocidal preparation of powdered infant formula - URL - note this covers all Cronobacter species, Salmonella and FAO-WHO Category B organisms (Enterobacteriacae and Acinetobacter)
- Genome sequence of C. sakazakii BAA-894 and genomic hybridisation comparison with other Cronobacter spp. in PLoS ONE 5(3): e9556 , 2010.
- Online 7 loci multilocus sequence typing (MLST):BMC Microbiology 2009. The MLST scheme is openly available at http://PubMLST.org/cronobacter.
- Phylogenetic, and phenotypic studies: J. Clin. Microbiol. 142:5368-70 (2004) and BMC Microbiology (Online) 6:28, and 6:94 (2006).
- Biofilms on neonatal enteral feeding tubes in intensive care: BMC Infectious Diseases 9:146 (2009) and laboratory studies Intl. J. Food Microbiol. 136: 227-231. (2009).
- Virulence: Food Microbiol. 24:67-74, 2007.
- General overview : Maternal and Child Nutrition 1:44-50, 2005. Voted UNICEF paper of the month.
- A risk profile of Cronobacter spp. in Trends in Food Science and Technology , 11:443-454, 2003.
- Development of a chromogenic agar called 'DFI' which stands for 'Druggan-Forsythe-Iversen agar; Int. J. Food Microbiol. 96:133-9, 2004. The agar has been commercialised by a number of companies; initially by Oxoid Thermo-Fischer (CM1055). An appraisal of enrichment media; Appl. Environ. Microbiol 73: 48-52, 2007.
- Growth range and thermotolerance Lett Appl Microbiol. 38:378-82, 2004.
- Survey of ~500 food products for Cronobacter spp. using the chromogenic agar compared with FDA method; Food Microbiology 21:771-6, 2004.
Our detection method is two days shorter than the original FDA method and is given here:
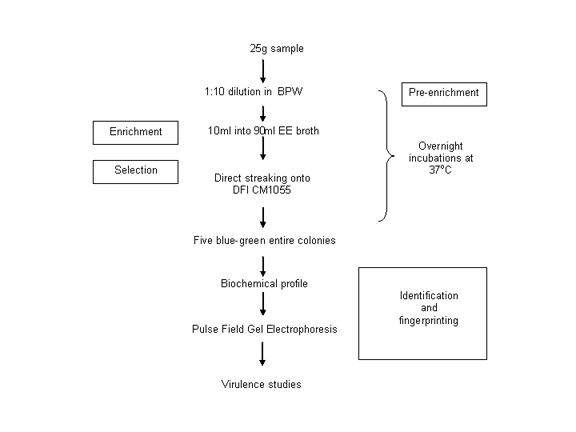
I have also been on various consultative panels concerning the organism. One such contribution being the three FAO-WHO risk assessment meetings (2004, 2006, 2008). The reports of both meetings are available at the FAO-WHO web site;
- WHO general factsheet on foodborne diseases
- WHO factsheet on medically important emerging diseases
- BSE - WHO factsheet
- vCJD - WHO factsheet
- CJD Surveillance Unit
- BSE-nvCJD
- Emerging pathogens in the UK
- Changes in consumer susceptibility
- Geographic distribution of vCJD
- Geographic distribution of vCJD, learnt?
- Multi-antibiotic resistant Salmonella in ground meat (US)
- European need to survey for antibiotic resistance
- ROAR Network
- WHO factsheet on human antibiotic usage
- WHO factsheet on antibiotic resistance
- Johne's - Crohn's disease and Mycobacterium paratuberulosis
- Assessment of surveillance and control of Johne's disease in farm animals in GB (214 page pdf file)
- Antimicrobial resistance report, UK FSA.
Other emergent pathogens
- Bacterial toxins; Friends or foes?
- E. coli, Salmonella and Shigella cytoskeletal protein toxins
Bacterial and fungal toxins
- Warning about eating beansprouts due to E. coli O157, written 2007
- List of outbreaks in North America due to beansprouts
Beansprouts
Outbreaks
Elsehwere there are links to commentary on the E. coli O104:H4 outbreak in Germany.
When an outbreak occurs, it needs to be investigated in a thorough, standardised manner. For a tutorial on this topic visit the CDC web page outbreak toolkit.
BSE-CJD news
The Department of Health in the United Kingdom continues to release the figures on both known cases of CJD and cases of variant Creutzfeldt-Jakob disease (vCJD)from 1990 web site. By the end of June 2002, a total of 124 cases of variant Creutzfeldt-Jakob disease (vCJD) had been reported in the United Kingdom. The overall median age at death was 28 (range 14-74 years). The median number of days from onset to diagnosis was 334 days and from onset to death 411 days. Of the 124 cases, 68 (55%) were male. It appears that the peak was in 2000. Twenty-eight cases of vCJD were reported in 2000, 15 in 1999, 18 in 1998, and 10 in 1997. Analyses shows that the underlying incidence was increasing by an estimated 18% per year based on date of symptom onset, or 20% per year based on date of death.
The FDA Bad Bug book
The Food and Drug administration (USA) have a very useful web site called Bad Bug Book which has extensive information on foodborne pathogens and toxins. You will notice that the majority of information in food microbiology is concerned with bacterial pathogens, as opposed to viruses and fungi. This is partially due to the relative ease with which bacteria can be cultivated in the laboratory. The perceived rise in viral causes in food poisoning is partially due to improved detection methods. The food poisoning statistics are dominated by Salmonella serotypes and Campylobacter jejuni. However as reviewed in foodborne illness the causative organism of a considerable number of gastroenteritis cases are not identified every year.
It should also be noted that a food microbiology in industry does not, and would not be expected to examine foods for all the possible pathogens. It would be prohibitively expensive and impractical. An additional reason for restricted microbiological analysis is that unless 100% of the food is examined then one cannot be 100% certain of the absence of pathogens or toxins in the whole batch of food. Taking known numbers of samples from a batch of food can be used to give a statistical evaluation of contamination. Hence the development of microbiological criteria. However it should be remembered that the results from a microbiological laboratory make take several days (at least if an outside accredited laboratory is used). Therefore the sampled batch of food might already have been distributed and a product recall would be expensive and deter customers from purchasing the product in the future.
Therefore the question arises as to how can a food manufacturer produce food that is microbiologically acceptable? The answer is in the proactive approach of Hazard Analysis Critical Control Point (HACCP). This internationally accepted means of assured safe food manufacture has already been used by large food companies for many years. However the implementation in small food outlets has been problematic due to the perceived burden of documentation. For assistance in industrial-scale HACCP implementation see the author's book Food Hygiene, Microbiology and HACCP.
